What is an Infusion Port (PORT)? An infusion port (PORT), also known as an implantable venous catheter system, is a fully implantable, closed venous infusion system. It is primarily used for cancer treatment, with about 80% of applications involving the infusion of chemotherapy drugs. It can also be used for intravenous infusions, parenteral nutrition, blood products, blood sampling, and more. The PORT can be used for the infusion of various types of drugs. A fully implantable infusion port, commonly referred to as a PORT, is a closed venous infusion system completely placed under the skin and designed to remain in the body long-term. It consists of a catheter inserted into the superior vena cava and an injection chamber implanted under…

An infusion port (PORT), also known as an implantable venous catheter system, is a fully implantable, closed venous infusion system. It is primarily used for cancer treatment, with about 80% of applications involving the infusion of chemotherapy drugs. It can also be used for intravenous infusions, parenteral nutrition, blood products, blood sampling, and more. The PORT can be used for the infusion of various types of drugs.
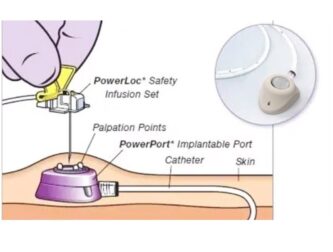
A fully implantable infusion port, commonly referred to as a PORT, is a closed venous infusion system completely placed under the skin and designed to remain in the body long-term. It consists of a catheter inserted into the superior vena cava and an injection chamber implanted under the skin. The catheter is placed via puncture of the internal jugular vein or subclavian vein, with the tip of the catheter reaching the superior vena cava. The port chamber is implanted in the subclavian region, connecting to the catheter, providing the patient with a long-term venous access channel. Depending on the implantation site, it can be classified into chest wall ports and arm ports, both suitable for drug infusion, fluid supplementation, nutritional support, blood transfusion, and blood sample collection.
Implantable-grade PEEK material offers excellent physical and chemical properties, with good radiolucency and biocompatibility. It has successfully replaced metals and other high-performance resins in fields such as orthopedic oncology, plastic surgery, spinal surgery, knee joints, and craniofacial reconstruction.
Compared to traditional infusion ports, AKSOPEEK® infusion ports offer the following advantages:
Excellent biocompatibility.
Low density — lighter and more comfortable to wear compared to metal infusion ports.
Good insulation properties — it does not easily retain heat when bathing or exposed to sunlight.
Intrinsic antibacterial properties — less likely to cause infection with long-term implantation, reducing complications.
Excellent corrosion resistance — unlikely to react with infused drugs or liquids.
Good radiation resistance — unaffected by radiotherapy or chemotherapy radiation.
Jiangsu Junhua is one of the earliest domestic enterprises engaged in the R&D and manufacturing of PEEK materials, with a strong focus on the application of PEEK in the medical industry. The company has been dedicated to the research, development, and production of implantable medical-grade PEEK materials and can supply implantable-grade PEEK granules, rods, plates, and 3D printing PEEK filaments.
After 16 years of production and technological accumulation, the company established Shandong Junhao High-Performance Polymers Co., Ltd., focusing on the polymerization of implantable PEEK raw materials starting from the source.
In compliance with the medical implant material production standards, a GMP purification workshop has been established, and specialized refining and purification equipment for AKSOPEEK® implantable-grade PEEK has been developed, effectively solving the issue of excessive heavy metal ion content in implantable PEEK materials.
In 2020, the company began pilot production of implantable-grade PEEK materials and conducted third-party biological testing in accordance with ISO 10993 standards. All test results met the required specifications.
In 2021, Junhua submitted the first batch of mass-produced implantable-grade AKSOPEEK® for third-party testing in accordance with the national standard YY/T 0660-2008: Standard Specification for Polyetheretherketone (PEEK) Polymers for Surgical Implants, and obtained comprehensive third-party test reports, ensuring the material’s reliable physical, chemical, and biological performance as a viable replacement.

With more than 18 years of experience in the application and development of polymer materials such as PEEK, Junhua Medical can support customers to complete one-stop services from raw material procurement to product OEM.
Get Quote
E-mail: peekmedica@chinapeek.com
WhatsApp: +8618136992681